Transcranial magnetic stimulation (TMS) was introduced in 1985 by Barker et al. as a non-invasive pain-free method to stimulate the human cortex (Barker et al., 1985). In their seminal communication, Barker et al. demonstrated that a single TMS pulse applied over the primary motor cortex (M1) elicits responses in those muscles that receive corticomotor input from the stimulated.1
For TMS, the stimulation device is discharged producing a strong time-varying magnetic field at right angles to the stimulation coil. The induced magnetic field reaches peak strengths of 1–2.5 Tesla and is very short lasting (⩽1 ms).1
What we understand by transcranial magnetic stimulation (TMS)
Transcranial magnetic stimulation (TMS) is a non-invasive treatment that involves using a magnetic coil to influence your brain’s natural electrical activity. Although developed in 1985, this technique now sees widespread use for a variety of mental health and brain-related conditions.
How a magnet influences brain function
Transcranial magnetic stimulation relies on two basic principles of physics: electricity and magnetism.
An electric pulse generator, or stimulator, is connected to a magnetic coil connected to the scalp. The stimulator generates a changing electric current within the coil which creates a varying magnetic field, inducing a current within a region in the brain itself.[1]
So, why does all that matter? It matters because human brain is electrically active. The brain cells in the brain and nerves (known as neurons) use tiny amounts of electricity to send and relay information throughout the brain and body. Bringing a magnet close to brain can influence brain’s electrical activity.
That’s why TMS targets specific parts of human brain, especially those related to emotions, internal decision-making, feelings of pleasure, etc.2
TMS has shown diagnostic and therapeutic potential in the central nervous system with a wide variety of disease states in neurology and mental health , with research still evolving.
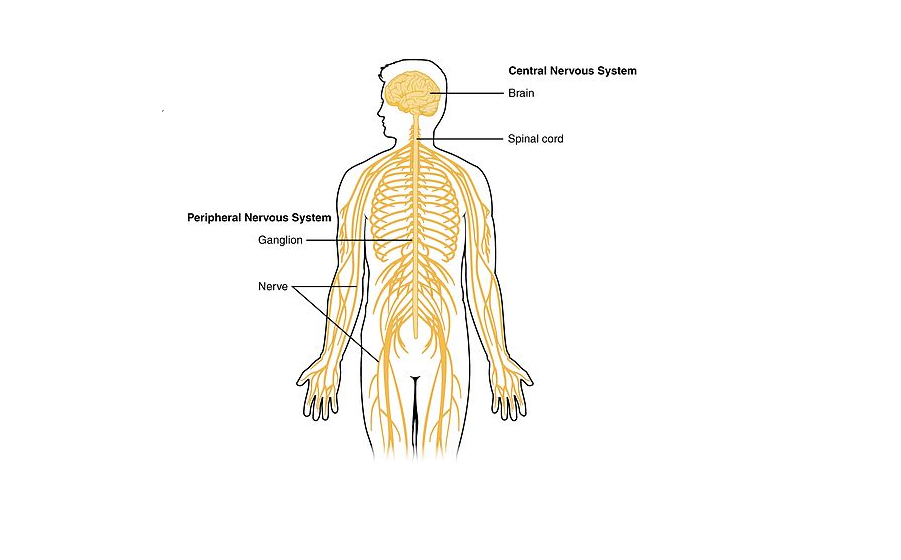
The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all parts of the bodies.
Therefore, TMS allows establishing causality between brain activations and different types of sensory, motor, and cognitive functions. TMS also be useful for diagnosis and treatment of some clinical conditions, such as migraine headache, depression etc., and for pre-surgical mapping of motor functions
How many types of TMS are available in the world
There are different ways to perform TMS. They have to do with the magnet’s strength or various ways to apply the magnetic field.
Magnet strength – The unit for measuring the strength of a magnet is the tesla (T). Most TMS magnets generate a magnetic field with a strength of 1.5T to 2T, similar to a magnetic resonance imaging (MRI) scanner. However, the area of the magnetic field is much smaller than it is for an MRI because the TMS magnet is so much smaller.
- Pulse frequency. Each time the magnetic field turns on and off is a pulse. The number of pulses per second is the frequency (which is measured in hertz, abbreviated Hz). TMS can involve low-frequency pulses at 1 Hz (1 pulse per second) or high-frequency pulses at 5 Hz to 10 Hz (5 pulses per second to 10 pulses per second). TMS that uses repetitive pulses is known as repetitive TMS (rTMS).
- Pulse patterns. TMS can also use different patterns of pulses for treatment. An example of this is theta-burst stimulation (TBS). During TBS, a triplet of 5 Hz bursts happens, for a total of 15 pulses in a second. Using these burst patterns speeds up treatment, making it about five or six times faster than other methods.
- Magnetic coil type and stimulation target. Different kinds of magnetic coils can target different brain structures. Deep TMS (dTMS), which involves an H-shaped helmed coil, targets deeper brain structures than rTMS and TBS. Research shows dTMS is effective in treating conditions such as obsessive-compulsive disorder(OCD).
So, there are four types of TMS – Single pulse, repetitive pulse known as repetitive pulse (rTMS), using different patterns of pulses known as theta-burst stimulation (TBS) and targeting deeper brain structure than rTMS known as dTMS
What is the difference between TMS, Deep TMS and rTMS
Transcranial magnetic stimulation (TMS) is a procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of major depression. It’s called a “noninvasive” procedure because it’s done without using surgery or cutting the skin. Approved by the U.S. Food and Drug Administration (FDA), TMS usually is used only when other depression treatments haven’t been effective.
“Deep transcranial magnetic stimulation” or “deep TMS.” Uses different type of coil. The deep TMS coil stimulates deeper and wider areas of the brain, compared with rTMS. Deep TMS coils have been FDA-approved for OCD and to stop smoking.
During an rTMS session, an electromagnetic coil is placed against the scalp of our head. This coil delivers magnetic pulses that stimulate nerve cells in a particular region that have decreased activity during depression. When used for depression, OCD and to stop smoking, this treatment involves delivering repeated magnetic pulses, so it’s called repetitive TMS or rTMS.
Why is transcranial magnetic stimulation is getting popular
It’s an important option because it’s non-invasive. That means it doesn’t require surgery, and the entire treatment happens through your skin.
Depression is a treatable condition. But for some people, standard treatments aren’t effective. Repetitive TMS may be used when standard treatments such as medicines, and talk therapy, known as psychotherapy, don’t work.
TMS is sometimes used to treat OCD, migraines and to help people stop smoking after other treatments have not been successful.
TMS can also offer an alternative to treatments like electroconvulsive therapy (ECT), if ECT isn’t an option or isn’t effective.
The conditions TMS has full approval to treat may vary from country to country. TMS has approval from the U.S. Food and Drug Administration (FDA) to treat four conditions:
- Depressive Disorder
- Major Depressive Disorder – MDD (including treatment-resistant depression).
- Obsessive-compulsive disorder (OCD).
- Migraines
- Smoking Cessation
In addition to the approved conditions, research is ongoing to see if it can treat other conditions. These include, but aren’t limited to:
- Addictions
- Alzheimer’s disease
- Bipolar disorder
- Borderline Personality Disorder
- Chronic Pain
- Eating Disorders
- Essential Tremor
- Fibromyalgia
- Parkinson’s Disease
- Post-traumatic stress disorder
- Schizophrenia
- Stroke complications.
- Tinnitus and auditory hallucinations
- Traumatic Brain injury
How often is transcranial magnetic stimulation performed
There’s limited available data on how many people undergo TMS per year. However, TMS is widely available, with accredited and certified providers in dozens of countries worldwide.
What happens before transcranial magnetic stimulation
Before you undergo TMS, a healthcare provider will probably refer you to a specialist with training and experience in using this treatment. The specialist will also talk to you before treatment to make sure you meet the criteria for having TMS or if there are any conditions or circumstances that mean you shouldn’t receive it.
Reasons that one shouldn’t undergo TMS can include:
- A history of seizures, epilepsy or other conditions/circumstances that affect your brain. While it’s rare, TMS can cause seizures. If you have a seizure-related condition or take a medication that increases the risk of seizure, your provider might recommend changing your medications or trying another treatment method altogether.2
- Pregnant or thinking of becoming pregnant
- Any kind of implant that’s electronic or contains magnetic metal. TMS is dangerous for anyone with cochlear implants, metal plates on their skull, etc. TMS involves a powerful magnet, which can pull on any magnetic metal in such implants. That pull could cause severe pain or even injuries if it moves an implant.
In some cases, people with metal implants or devices can have rTMS. But due to the strong magnetic field produced during rTMS, it’s not recommended for some people who have these devices:
- Aneurysm clips or coils.
- Stents
- Implanted stimulators.
- Implanted vagus nerve or deep brain stimulators.
- Implanted electrical devices, such as pacemakers or medicine pumps.
- Electrodes for monitoring brain activity.
- Cochlear implants for hearing.
- Magnetic implants.
- Bullet fragments.
- Other metal devices or objects implanted in their body
- taking medicines,including prescriptions, medicines available without a prescription, herbal supplements, vitamins or other supplements, and the doses.
- have a history of seizuresor a family history of epilepsy.
- have other mental health conditions,such as issues with alcohol or drugs, bipolar disorder, or psychosis.
- have brain damage from illness or injury,such as a brain tumour, stroke or traumatic brain injury.
- have frequent or severe headaches.
- have any other medical conditions.
- Already had treatment with rTMSin the past and whether it was helpful in treating your depression.
If your provider determines that TMS is a good and safe option for you, they’ll talk with you about the treatment schedule they recommend. TMS takes multiple treatments — typically three to five per week — spread out over several weeks. For FDA-approved protocols for depression, a typical TMS course happens every weekday over six weeks for a total of 30 treatments. However, some newer treatment courses can greatly reduce the course of treatment down to a single week.
Because there’s some variability in treatment courses, your provider is the best person to explain the recommended course of treatment sessions. They can provide the most accurate information specific to your case.2,6
What happens after TMS
Once a treatment session is over, you can return to your usual routine or schedule for the day. If you have lingering side effects, such as twitching or unusual sensations in your head or face, your provider may ask you to wait a few minutes before leaving. Most of the symptoms that follow a treatment session are mild and only last a few minutes (more about these under the Risks and Benefits section).
What Are the Age Limits to Using TMS Therapy
The Food and Drug Administration (FDA) has approved TMS for individuals between the ages of 18 to 70
[ A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation ]
However, numerous clinical studies have shown that TMS is both safe and effective at treating various conditions in children as young as 9 years old, including:
-
-
- Depression
- Mood disorders
- Autism spectrum disorder (ASD)
- Attention deficit hyperactivity disorder (ADHD)
-
Research further indicates that children and adolescents with mental health conditions who receive as few as 10 TMS sessions for at least two weeks experience improved symptoms [8]. Most youths demonstrate reduced depression, irritability, and attention deficits after TMS treatment.7, 9. Although TMS for children is not yet approved by the FDA, it is a beneficial alternative for parents to consider. 7
Applications of transcranial magnetic stimulation (TMS) in child and adolescent psychiatry
Transcranial magnetic stimulation (TMS) is emerging as a new treatment and neurophysiological research tool for psychiatric disorders. Recent publications suggest that this modality will also serve as a treatment and research tool in child and adolescent psychiatry. 7
Is TMS Therapy Safe for Children
Clinical reviews suggest that the risks and side effects of TMS therapy are the same for both children and adults. 8,9
One large-scale review of 384 children (1 to 18 years) who received TMS therapy over the course of 10 years provides evidence of the safety and tolerability of TMS for kids. According to the findings, no serious side effects occurred and the rate of seizures due to TMS was negligible even in children diagnosed with epilepsy or traumatic brain injuries (TBIs). 10,11
Mild headache is one of the only common side effects, but it is typically temporary and resolves without needing treatment.
Based on the favourable results, researchers recommend that healthcare professionals who provide TMS for children follow the most recent safety guidelines established for adults until additional clinical evidence is available for pediatric-specific TMS guidelines.10,11
iTBS vs TMS: What is the Difference
TMS is a therapeutic approach towards treating mental illnesses by stimulating areas of the brain that are associated with mood. Whereas electroencephalography (EEG) report, is essentially a thermal map of the brain — representing electric activity happening in neurons.
Theta burst stimulation is essentially a new protocol of TMS that allows different frequencies of magnetic stimulation, allowing for a shorter treatment time and potentially better treatment results for a range of different illnesses.
How does TMS and TBS differ?
Theta burst stimulation form of transcranial magnetic stimulation that can be delivered in 3 minutes rather than 30-45 minutes with rTMS.
The two popular theta burst stimulation protocols are intermittent theta burst stimulation and continuous theta burst stimulation:
- Intermittent theta burst stimulation (iTBS) is an excitatory stimulation that essentially activates neurons that are underactive, such as seen in depression.
- Continuous theta burst stimulation (cTBS) is an inhibitory stimulation that is used to decrease neuronal activity in overactive parts of the brain, such as seen in anxiety or PTSD.
The difference between these two types of theta burst protocols and normal repetitive transcranial magnetic stimulation is the frequency of electromagnetic stimulation.
Standard TMS typically runs at 10 Hz. And, the typical theta burst stimulation protocols run at 50 Hz every 200 ms. With iTBS, this stimulation runs for 2 seconds on, and 8 seconds off. Typically, both run for 600 pulses within 3 minutes. With cTBS, that stimulation is continuous and actually calms parts of the brain.
References
[1] National Library of Medicine, A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee
[2] Transcranial Magnetic Stimulation (TMS),Cleveland Clinic
[3] Magnetic stimulation: a new approach to treating depression, Michael Craig Miller, M.D., Harvard Health Publishing
[4] Transcranial magnetic stimulation for treating and preventing migraine, National Institute for Health and Care Excellence
[5] Clinically Meaningful Efficacy and Acceptability of Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) for Treating Primary Major Depression: A Meta-Analysis of Randomized, Double-Blind and Sham-Controlled Trials; National Library of Medicine
[6] Transcranial Magnetic Stimulation , Mayo clinic
[7] Paul E Croarkin 1, Christopher A Wall, Jon Lee, Pub med, NLM
[8] BRIEF RESEARCH REPORT article, Front. Psychiatry, 29 March 2019, Sec. Neuroimaging,
Volume 10, Frontiers
[9] Innov Clin Neurosci. 2019 Sep 1; 16(9-10): 33–35. Published online Sep-Oct 2019.
Puneet Narang, MD, Katelyn Madigan, BS, Simrat Sarai, MD, and Steven Lippmann, MD
[10] Safety and tolerability of transcranial magnetic and direct current stimulation in children,
Pub med, NLM
[11] Walter G. Griffith Jr., MD, PA, Dr. TMS therapy.

Debasis Chudhuri

